lateral flow assay protocol
Assay Procedure 7 6. Universal Lateral Flow Assay Kit ab270537 is designed to enable simple and rapid development of proof-of-concept sandwich lateral flow immunoassays.

Lateral Flow Webinar A Guide To Lateral Flow Immunoassay Development Youtube
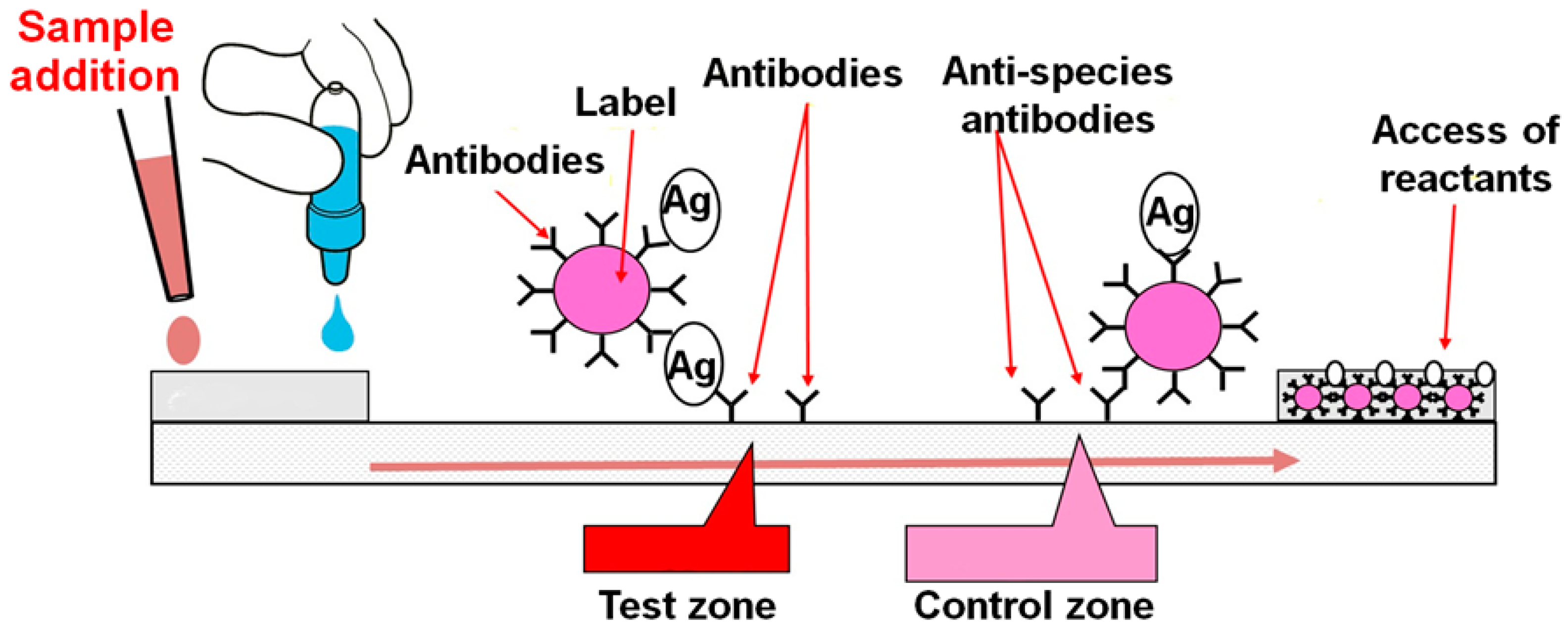
The lateral flow assay LFA is a paper-based platform for the detection and quantification of analytes in complex mixtures where the sample is placed on a test device and the results are displayed within 530 min.
. The various types of formats excellent features on field applicability of these strips have made them an ideal choice for point of care applications like testing of pathogens metabolites hormones allergens drugs etc. Recent technology developments permit the use of inexpensive electronic readers for interrogating lateral flow strip test results thus avoiding the inevitable variation and subjectivity of visual inspection to assess the capture of reporter-labeled analyte on. DHSC will identify a pipeline of products direct viral antigen detection lateral flow devices and distributed amplification tests that could enable saturation testing for SARS-CoV-2.
Coronavirus Lateral Flow Assay LFA sample preparation protocol. The sample flows from the sample padblood separator through to. These assays have the benefit of being portable fast and relatively inexpensive.
822 Remove the protective foil cover from each test. Throughout the previous courses of the NCXU program we have discussed many critical components of a lateral flow assay. Lateral flow assays LFAs are simple to use disposable diagnostic devices that can test for biomarkers in samples such as saliva blood urine and food.
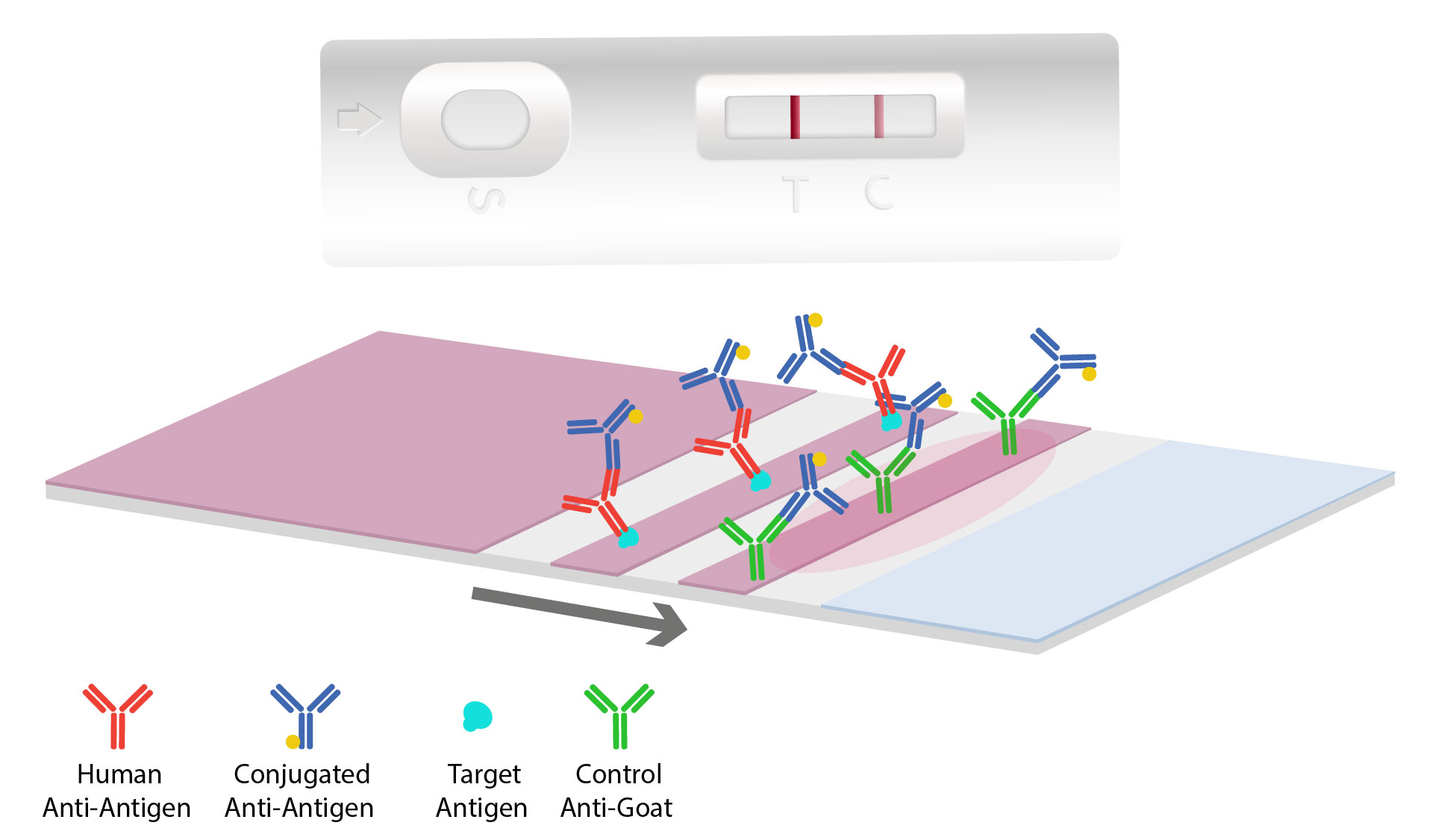
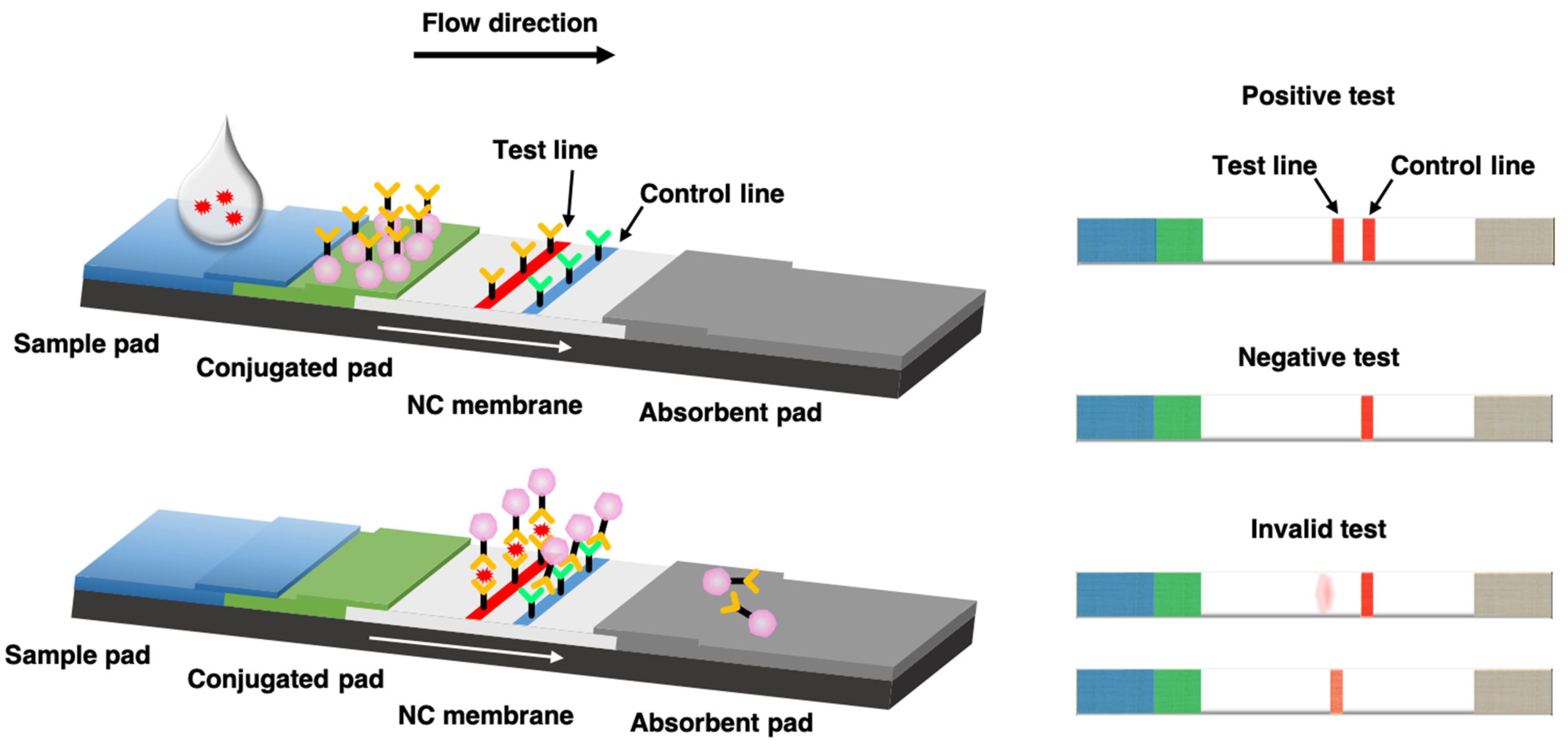
Overview Universal Lateral Flow Assay Kit ab270537 is designed to enable the easy development of customized sandwich lateral flow assays by combining Lightning-Link and Gold conjugation technologies with an immunochromatography test performed on Universal-LFA. The lateral flow assay LFA is a paper-based platform for the detection and quantification of analytes in complex mixtures where the sample is placed on a test device and the results are displayed within 530 min. The darker red line indicates that the test was valid.
Up to 10 cash back Lateral flow assays are being used for various quantitative and qualitative analyses in a number of areas. It combines universal LFA strips Lightning-Link and. Removal of the test units should start from the right side of the test card to preserve the lot number which appears on the left side of the test card.
Low development costs and ease of production of LFAs have resulted in the expansion of its applications to multiple fields in which. Schematic of typical lateral flow assay. Range than is needed from a qualitative assay.
Assay Procedure 7 6. Tearing at the perforation. In response to the COVID-19 pandemic lateral flow assays LFAs for the detection of SARS-CoV-2 antigen have been proposed as a complementary option to the more costly and time consuming reverse.
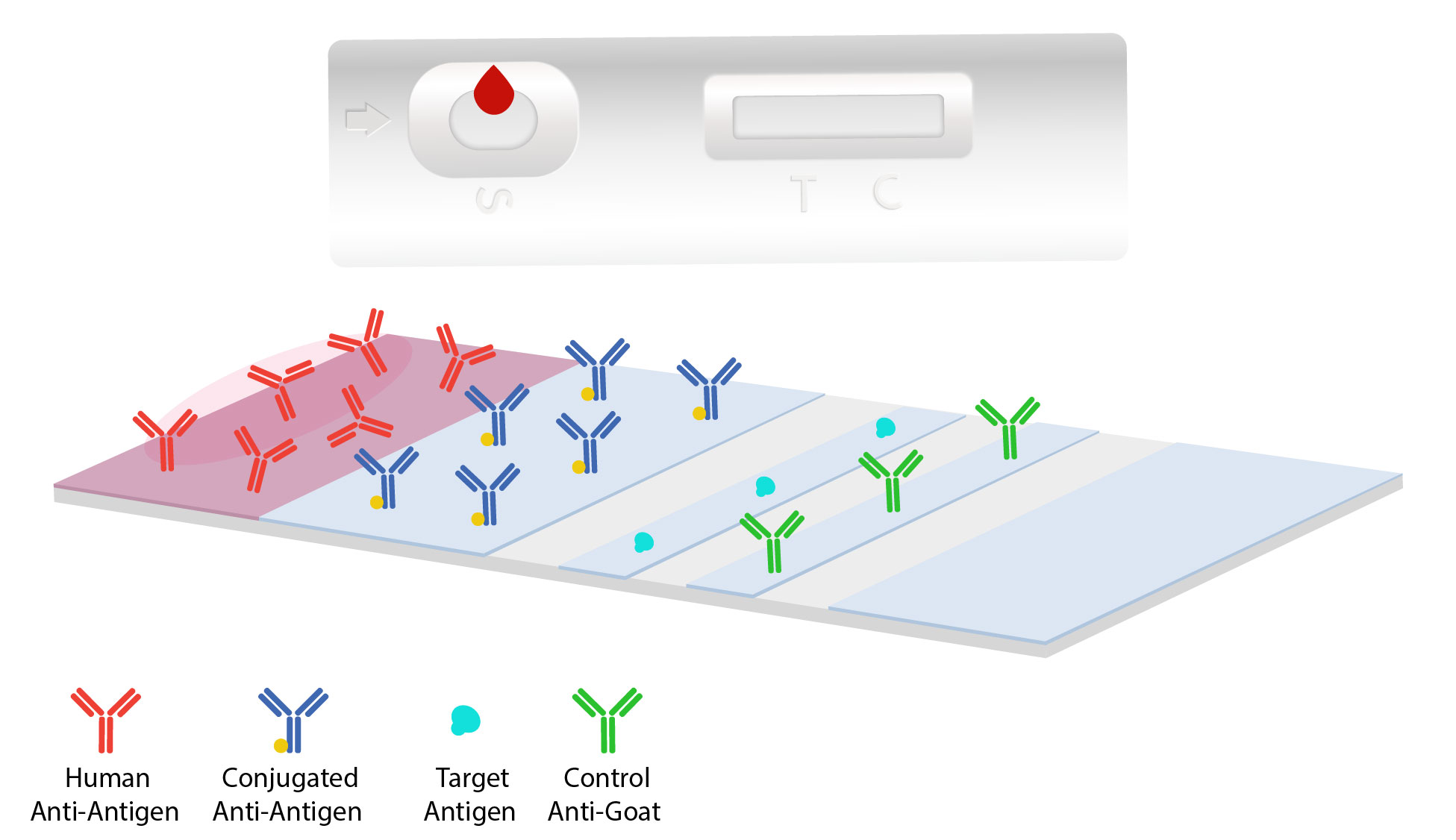
Although there are a number of different variations of the technology they all operate using the same basic. A lateral flow test LFT is an Assay also known as a lateral flow device LFD lateral flow immunochromatographic assay or rapid test is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipmentLFTs are widely used in medical diagnostics in the home at the point of care and in. The faint red line indicates that the subject is pregnant.
Principles and characteristics of lateral flow strip assays are reviewed. Hence a meta-analysis would be performed to evaluate the accuracy of LFA in detecting influenza virus. It combines universal LFA strips Lightning-Link and.
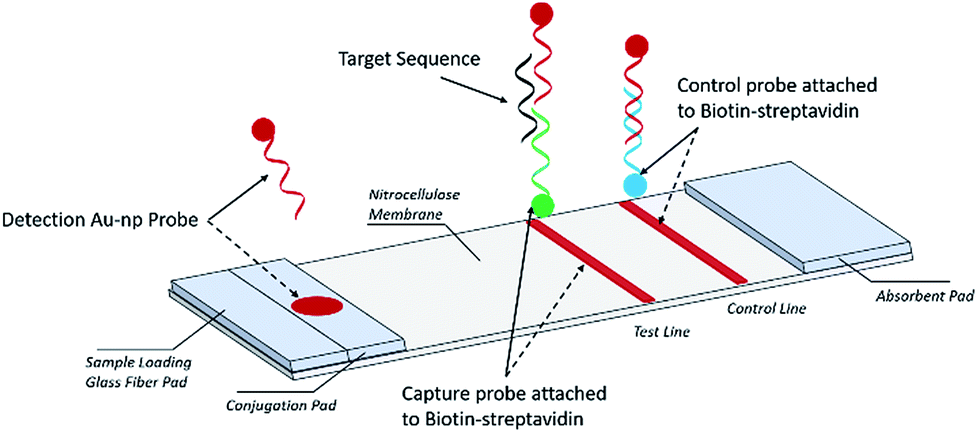
I also read MERCKs guideline Rapid Lateral Flow Test Strips and it said that 005 SDS or 0005 Tritonx-100 should be added to the capture reagent solution to. It is based on the movement of sample across the membrane via capillary force. Since the gold nanoshell has the same gold surface as smaller solid gold nanoparticles only minor protocol modifications are required to switch from solid gold nanospheres to gold.
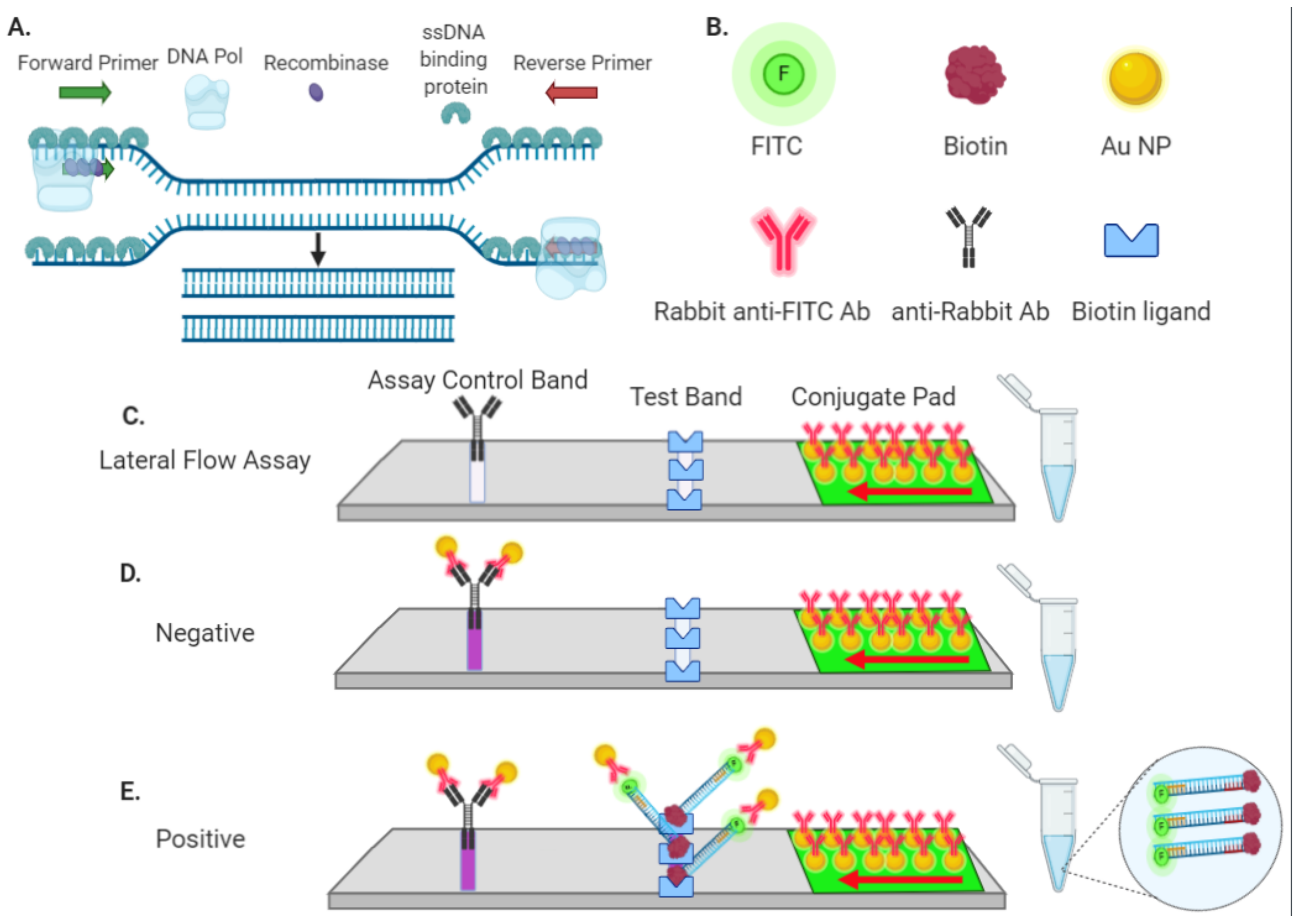
A lateral flow assay is composed of a chromatographic system separation of components of a mixture on the basis of differences in their movement through reaction membrane and immunochemical reaction between antibody-antigen nucleic acid-target analyte. Lateral flow immunoassays are qualitative POC tests that use antibodies to a protein of interest in any of a variety of bodily fluid samples including blood and saliva Carter et al 2020. The kit includes everything needed for lateral flow assay development with any pair of capture and detection antibodies.
Few assays begin with the target analytical range sensitivity or clinical performance without issues that need to be resolved. A commercial pregnancy test which uses 40 nm gold nanoparticles as the detection label. The most commonly known type of lateral flow rapid test strip is the pregnancy test.
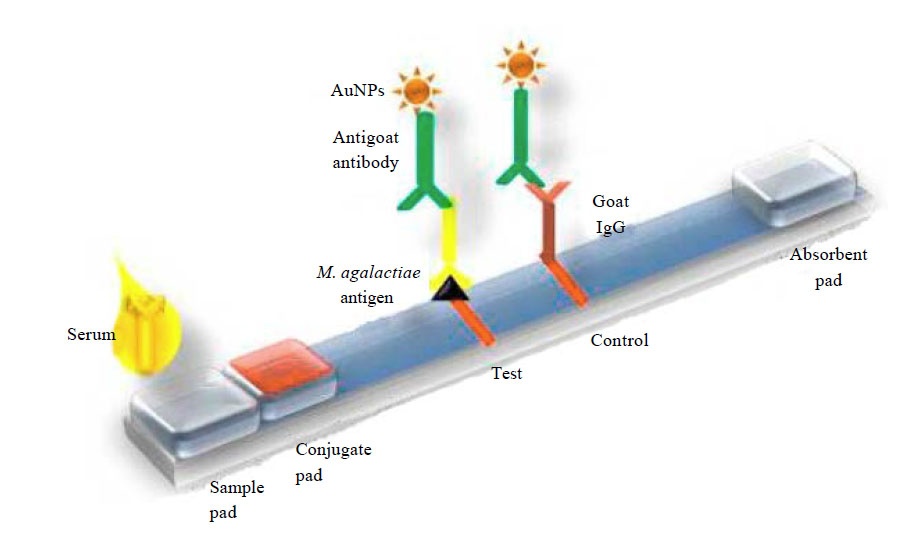
Lateral flow immunoassays Lateral flowimmunoassays also known as immunochromatographic assays or strip tests are immunoassays which have been designed to operate along a single axis. Basically it is a simple to use diagnostic device used to confirm the presence or absence of a target analyte such as pathogens or biomarkers in humans or animals or contaminants in water supplies foodstuffs or animal feeds. Lateral flow immunoassay systems are generally single-step assays requiring only the addition of a sample.
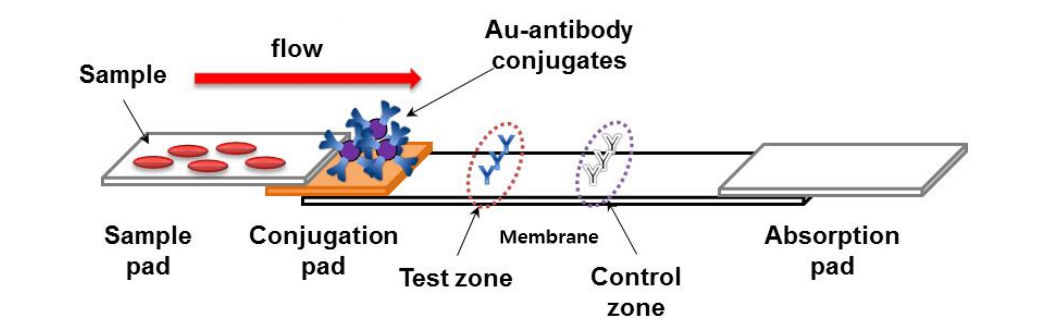
They consist of a sample pad or blood separator conjugate release nitrocellulose membrane and an absorption pad Fig 1. But the effectiveness of the technique in detecting flu viruses is unclear. Each component requires systematic optimization in order to develop a precise and accurate test.
Macbook pro touch bar sensitivity. The assay should be initiated within 2 hours after removing the protective foil cover from each test. Introduction to Lateral Flow OVERVIEW Figure 1 Figure 1.
The simplicity and low cost of lateral-flow assays LFAs have made them one of the most used point-of-care PoC sensors 12 in various disciplines ranging from. Cite this protocol as. The lateral-flow assay is a rapid method to detect influenza virus.
91 81180 50960 infotheklovein funny gifts for coffee lovers. Lateral Flow Assay Optimization 10 7.
Molecular Diagnostics Lateral Flow Assay

Lateral Flow Assay Schematic Design A A Lateral Flow Test Strip Download Scientific Diagram

Universal Lateral Flow Assay Kit Ab270537 Abcam

Simple Lateral Flow Assays For Microbial Detection In Stool Analytical Methods Rsc Publishing Doi 10 1039 C8ay01475b
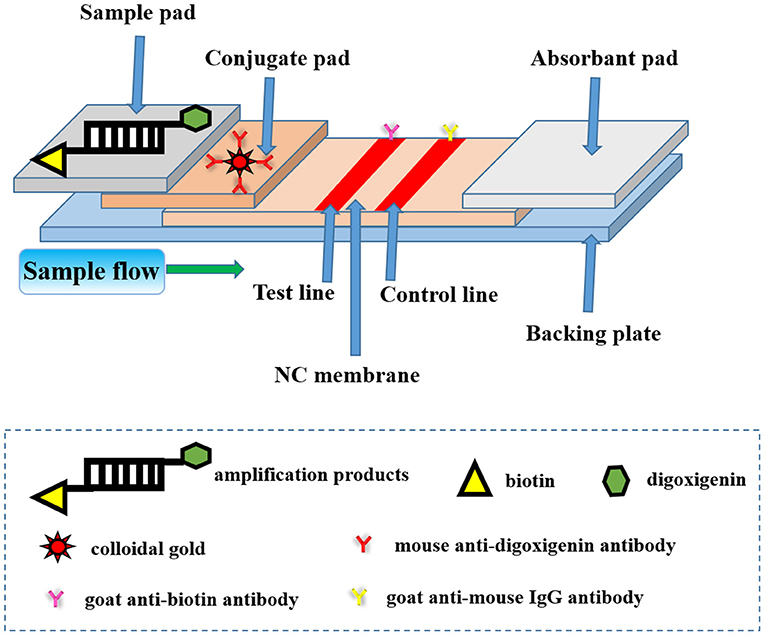
Frontiers A Novel Lateral Flow Assay For Rapid And Sensitive Nucleic Acid Detection Of Avibacterium Paragallinarum Veterinary Science
Biosensors Free Full Text Towards Lateral Flow Quantitative Assays Detection Approaches Html
Lateral Flow Assay Development Leinco Technologies

Principle Of Competitive Lateral Flow Immunoassays The Device Is Download Scientific Diagram

Steps In Lateral Flow Immunoassay Lfia Based Covid 19 Diagnosis A Download Scientific Diagram
Bioengineering Free Full Text Crispr Cas9 Based Lateral Flow And Fluorescence Diagnostics Html
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html
Lateral Flow Immunoassay Creative Diagnostics

Schematic Of A Lateral Flow Assay With Colloidal Gold As Label Download Scientific Diagram
Lateral Flow Rapid Test Assay Optimization Nanocomposix

Tutorial Design And Fabrication Of Nanoparticle Based Lateral Flow Immunoassays Nature Protocols

Lateral Flow Test An Overview Sciencedirect Topics

Capillary Flow Control In Lateral Flow Assays Via Delaminating Timers

Review Of Recent Advances In Improved Lateral Flow Immunoassay For The Detection Of Pathogenic Escherichia Coli O157 H7 In Foods Sun 2021 Journal Of Food Safety Wiley Online Library